AmnioAmp MP
Q4250* Pricing may vary based on quantity and specific requirements. Contact us for detailed pricing information.
Product Description
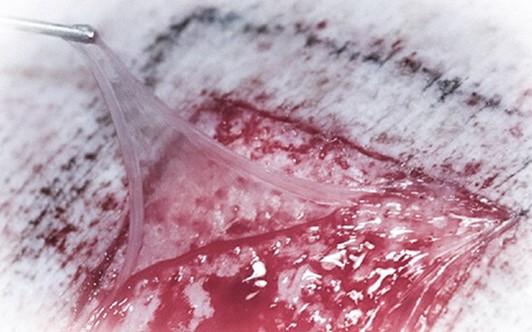
AmnioAMP-MP™ is a membrane allograft that may be used as a therapeutic active barrier agent in numerous clinical applications. The membrane forms a protective covering over the wound to preserve tissue structure and biologic integrity. AmnioAMP-MP™ created with our proprietary PūrAMP Process™, offers mechanical protection and retains nutrient growth factors that are essential for signaling¹². The product undergoes active preservation of the extracellular matrix (ECM), regulatory proteins, and removal of blood contaminants via a proprietary PūrAMP Process™. AmnioAMP-MP™ is HCT/P compliant under section 361 of the Public Health Service Act according to 21 CFR Part 1271.10. This is regulated as human cell product-for homologous use only under level II.
Key Features & Benefits
- Natural Adherence – Adheres naturally to the patient’s tissues without need for sutures or other fixation
- Multi-directional – can be applied in either direction, with no orientation restrictions
- Stored at ambient temperature (50-86ºF/10-30ºC) until ready for use
- Ease of placement due to patent PūrAMP preservation technology
- 1 year shelf life
Sizing Options
| Size | SKU | Description |
|---|---|---|
| 2x2 | CG1102 |
AmnioAmp MP |
| 2x3 | CG1100 |
AmnioAmp MP |
| 2x4 | CG1101 |
AmnioAmp MP |
| 4x4 | CG1104 |
AmnioAmp MP |
| 4x6 | CG1105 |
AmnioAmp MP |
| 4x8 | CG1106 |
AmnioAmp MP |
| 8x16 | CG1107 |
AmnioAmp MP |
| 9x20 | CG1108 |
AmnioAmp MP |
| 12x21 | CG1109 |
AmnioAmp MP |
Product Documentation
Product Brochure
Download our comprehensive product brochure with detailed information and specifications.
Download BrochureInstructions for Use (IFU)
Download the official Instructions for Use document with handling and application guidelines.
Download IFUTechnical Specifications
Product Type
Thick, dual-layer membrane
HCPCS Code
Q4250
Pricing
$2,901.65
Storage
Room Temperature, Sterile Packaging
Frequently Asked Questions
Our tissue allografts are processed and packaged to maintain stability at room temperature. Each product comes with specific expiration dating and storage instructions to ensure optimal performance and safety.
To order our products, please contact our sales team directly. We work with healthcare providers to ensure proper ordering, delivery, and support for all our tissue allograft products.
We provide comprehensive training and support materials for healthcare professionals. This includes product handling instructions, application techniques, and clinical best practices to ensure optimal outcomes.
Let's Build Healthier Solutions Together
Get in touch with our team to discuss your healthcare needs